ATOMIC FLUORESCENCE SPECTROMETRY (AFS)
Basic Theory
AFS is a two stage process of excitation and emission
Stage 1: A high intensity monochromatic discharge lamp provides the excitation energy which is focused onto the analyte atoms.
Stage 2: The electrons surrounding the atom absorb the energy and are excited to a higher energy level. This is an unstable state and the electron quickly drops back to it's original ground state. In doing so, the election loses the energy in the form of emitted light (fluorescence).
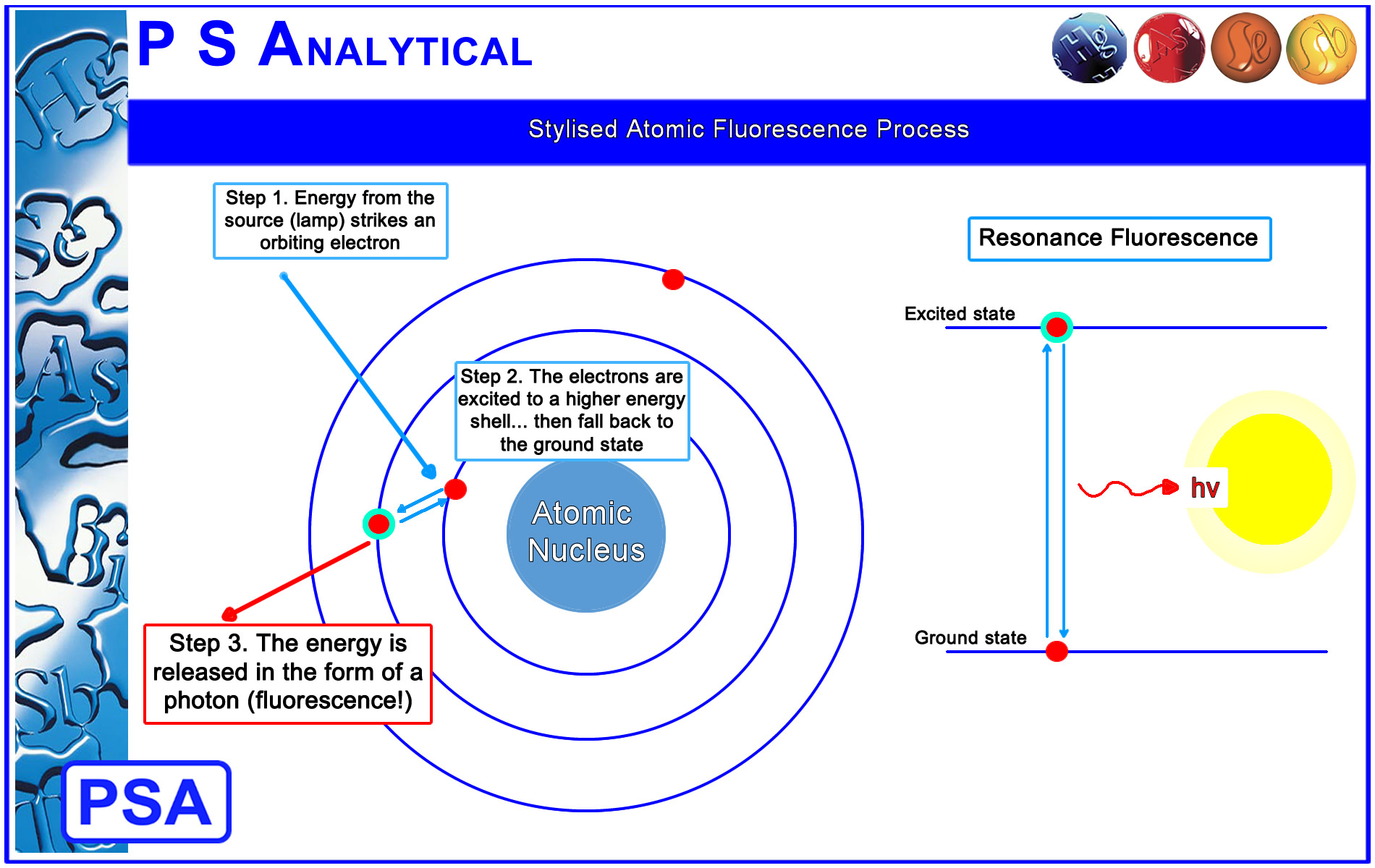 |
|
A couple of key points:
1. Since atomic species do not have vibrational energy levels, the emitted photons are at the same wavelength as the incident radiation. This process of re-emitting the absorbed photon is "resonance fluorescence" and is characteristic of atomic fluorescence.
This is sometimes referred to as the "lock and key" effect.
It means that if you want to determine mercury by AFS you MUST employ a mercury lamp; aresnic, an arsenic lamp and so on.
Due to this fact the technique is element specific and is much less subject to interferences from the presence of other elements.
2. The intensity of the fluorescence emitted in directly proportional to the amount of excitation energy provided.
High energy discharge lamps or boosted hollow cathode lamps should be used to get the best sensitivity. AFS analyzers have exceptional limits of detection.
Being an emission method AFS also benefits from wide dynamic linear working ranges.
If you require any further information, please fill in our Information Request Page.




